Transport by biological nanomachines.
The highly efficient molecular devices that conduct molecular transport in living cells have evolved to optimize cell survival and function. Their efficiency, selectivity and resilience to noise and structural damage inspire designs of biomimetic devices for biotechnology and biomedical applications. One fundamental question has been the long term focus of our research: how can the function of these "nanomachines” be so precise on the nanoscale in presence of high levels of non-specific interactions in the crowded and thermally noisy cell environment?
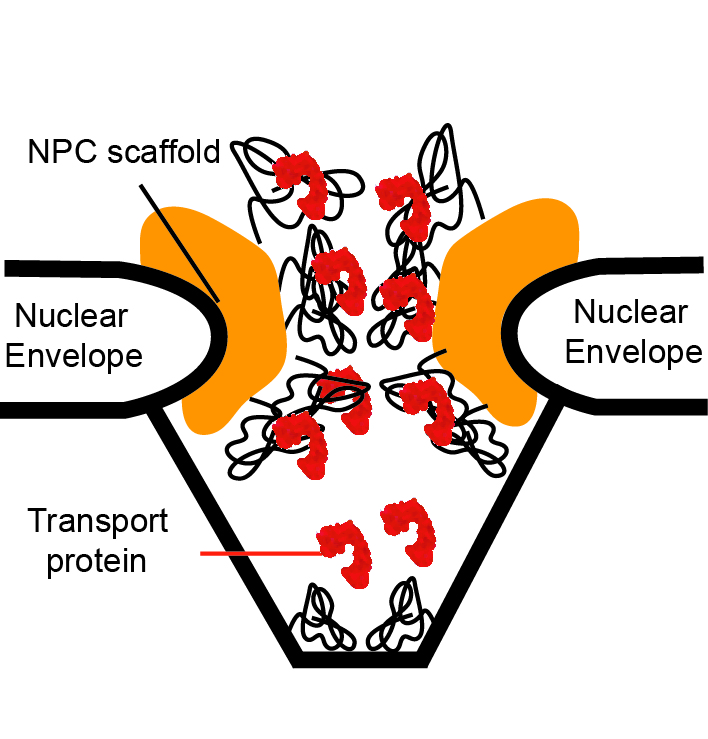
One of our term focus areas has been the biophysics of the Nuclear Pore Complex (NPC) - “biomachine” that controls the nucleocytoplasmic transport in all eukaryotic cells and is central for a large number of regulatory and disease processes. Understanding the unique architecture and transport mechanisms of the NPC holds the keys to a large number of puzzles and practical applications, such as the design of artificial macromolecular sorting devices. We use theoretical models and simulations on various scales to understand the principles of its function.
One of our term focus areas has been the biophysics of the Nuclear Pore Complex (NPC) - “biomachine” that controls the nucleocytoplasmic transport in all eukaryotic cells and is central for a large number of regulatory and disease processes. Understanding the unique architecture and transport mechanisms of the NPC holds the keys to a large number of puzzles and practical applications, such as the design of artificial macromolecular sorting devices. We use theoretical models and simulations on various scales to understand the principles of its function.
Intrinsically disordered proteins and other polymeric materials on the nanoscaler.
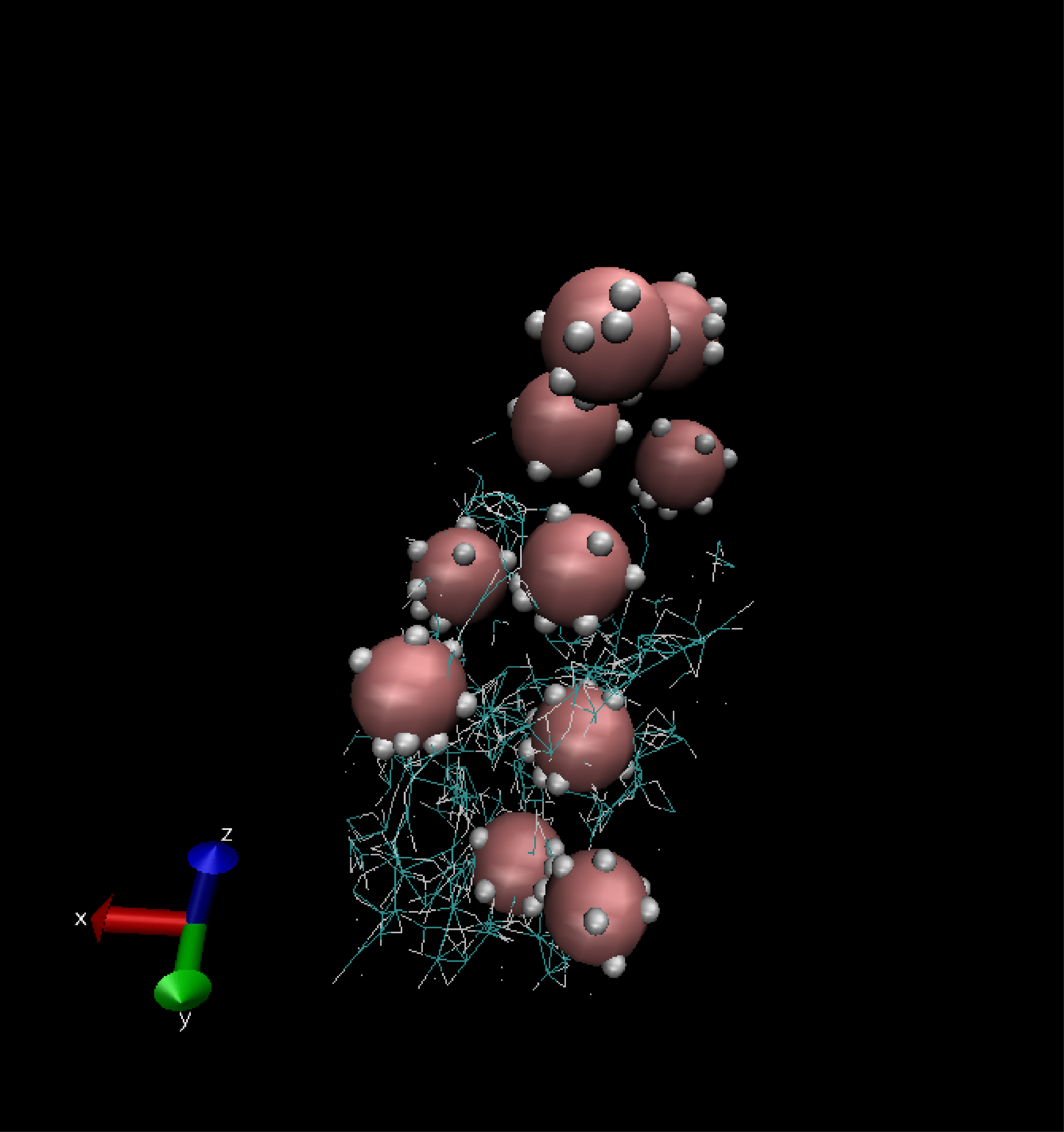
The key component of the NPC transport mechanism of the NPC is the assembly of intrinsically disordered proteins that gates its passageway. Although the properties of these intriniscally disordered proteins have been hypothesized to be crucial for the NPC transport properties, their collective morphologies and dynamics remain unknown. A major impediment is the paucity of experimental methods that can probe their conformational dynamics in vivo on the relevant time and length scales. In vitro experiments provide apparently contradictory results that have led to conflicting theories. This has brought the computational analyses to the forefront of the research in this area. Our collaborative group that includes theorists from the University of Pittsburgh and experimentalists from the University of Basel and Texas A&M has been developing coarse grained models of the assemblies of these polymer-like proteins rooted in statistical physics of soft matter. Beyond the NPC, these models are important for the understanding of the morphologies of nanoparticle-polymer assemblies used in the design and control of “smart” nanomaterials such as molecular coatings, sensors, nano-valves, nano-carriers for drug delivery, and other applications.
Immunological signaling. Many of the fundamental questions of specificity, efficiency and resilience to noise and structural damage that arise in the context of transport “machines” also feature in the multitude of signaling processes in the cell. In addition to numerous non-specific interactions, many signaling pathways act through shared components, where different ligand molecules bind the same receptors or activate overlapping sets of response regulators downstream. Nevertheless, different ligands acting through cross-wired pathways often lead to different outcomes in terms of the target cell behavior and function. How multicellular organisms can maintain precise control of signaling networks involved in multiple processes, remains a puzzle.
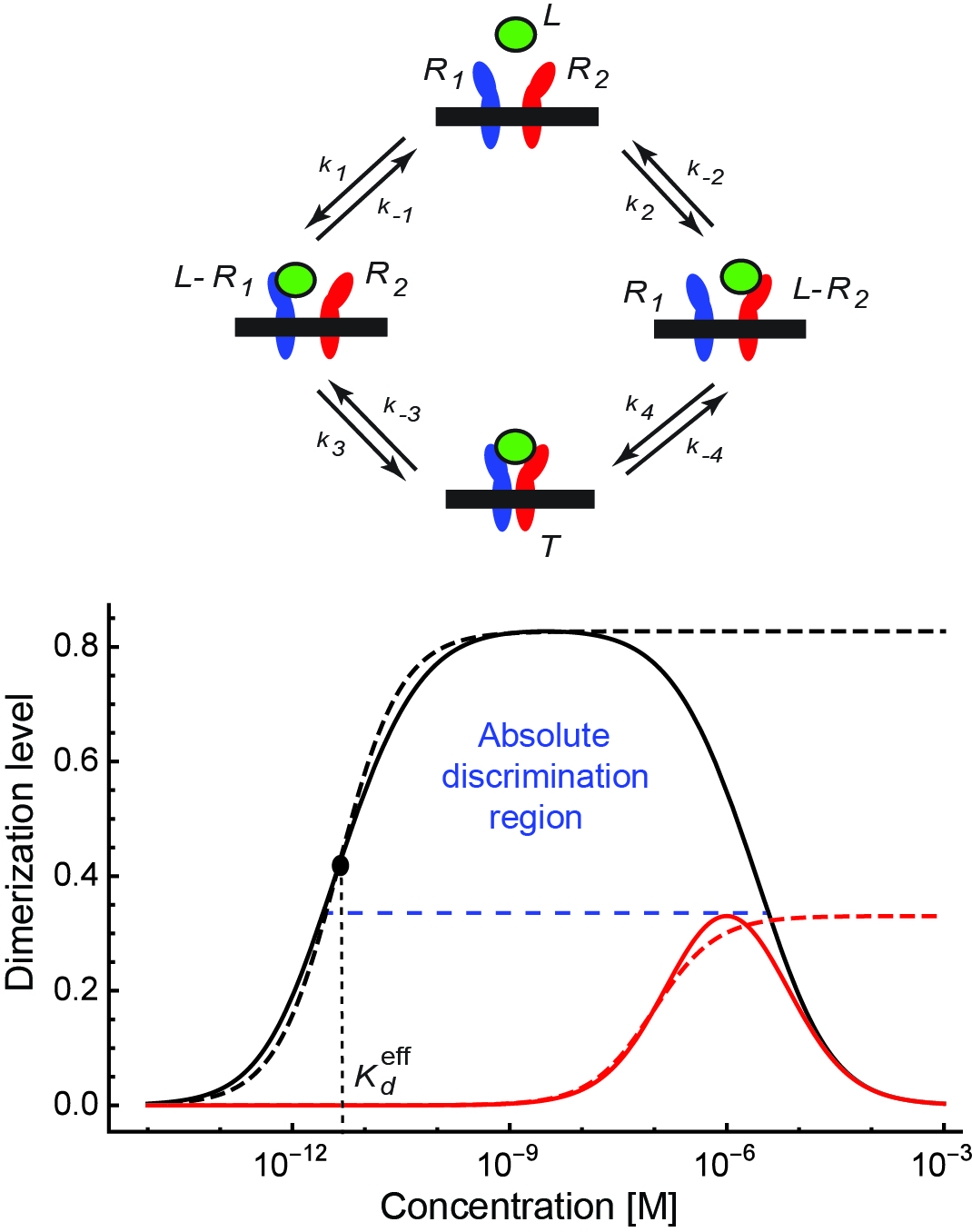
We have been focusing on the specificity and cross-talk in signaling by Type I Interferons - a major class of signaling molecules of the innate immune system, which provides the first line of defense of higher organisms against pathogens. Type I Interferon signaling is a striking example of the specificity problem: a large number of different Interferons act through the same receptor but elicit different responses in the target cells. They also exhibit a puzzling pattern of temporal cross-talk , which poses a problem for their clinical uses in the treatment of a number of diseases, including Hepatitis C, Multiple Sclerosis and several cancers. To answer these fundamental and applied questions, we have built a quantitative and physically rigorous model of specificity, cross-talk and adaptation in Interferon receptor signaling, informed by experimental data. We have shown how the specificity and cross-talk naturally arise from the kinetics of the Interferon receptor dimerization. The model addresses several outstanding experimental puzzles and directly affects the clinical use of Type I Interferons in treatment of viral hepatitis and other diseases.
We have been focusing on the specificity and cross-talk in signaling by Type I Interferons - a major class of signaling molecules of the innate immune system, which provides the first line of defense of higher organisms against pathogens. Type I Interferon signaling is a striking example of the specificity problem: a large number of different Interferons act through the same receptor but elicit different responses in the target cells. They also exhibit a puzzling pattern of temporal cross-talk , which poses a problem for their clinical uses in the treatment of a number of diseases, including Hepatitis C, Multiple Sclerosis and several cancers. To answer these fundamental and applied questions, we have built a quantitative and physically rigorous model of specificity, cross-talk and adaptation in Interferon receptor signaling, informed by experimental data. We have shown how the specificity and cross-talk naturally arise from the kinetics of the Interferon receptor dimerization. The model addresses several outstanding experimental puzzles and directly affects the clinical use of Type I Interferons in treatment of viral hepatitis and other diseases.
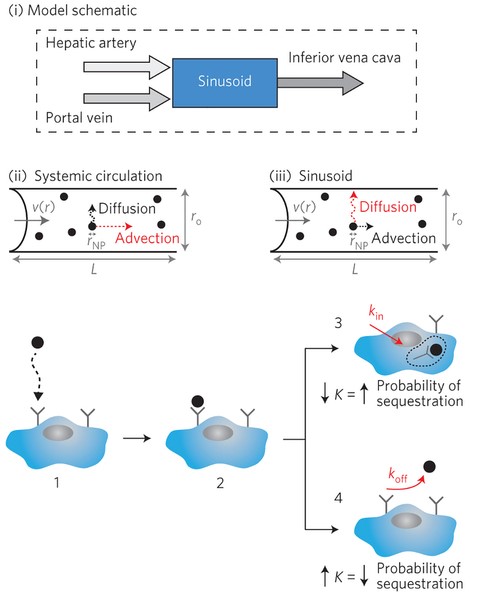
Targeted drug delivery.. To achieve the long-sought goal of drug delivery targeted directly to affected organs and cells, intensive efforts have been invested in the design of nano-size drug carriers functionalized by ligands that home them to specific organs or cell types. However, the success of such treatments is impeded by vast non-specific interactions and adsorption of blood proteins onto the nanocarriers, and their subsequent sequestration by the cells of the immune system - predominantly in the liver. Prevention of this sequestration requires understanding the physics of nanocarrier transport in blood and their interactions with different organs, as a major stepping stone for practical use of nanocarriers for drug delivery. We are addressing these issues within a large collaborative effort with Dr. Warren Chan at IBBME at the University of Toronto, an expert in the nano-carrier development, and Dr. Ian McGilvray at the Toronto General Hospital - a surgeon specializing in liver surgery. We have developed the first rigorous mathematical model of nano-carrier transport in blood through liver passages, known as sinusoids. This work is the first step in the direction of rational development of drug delivery carriers.
Stochastic population dynamics.
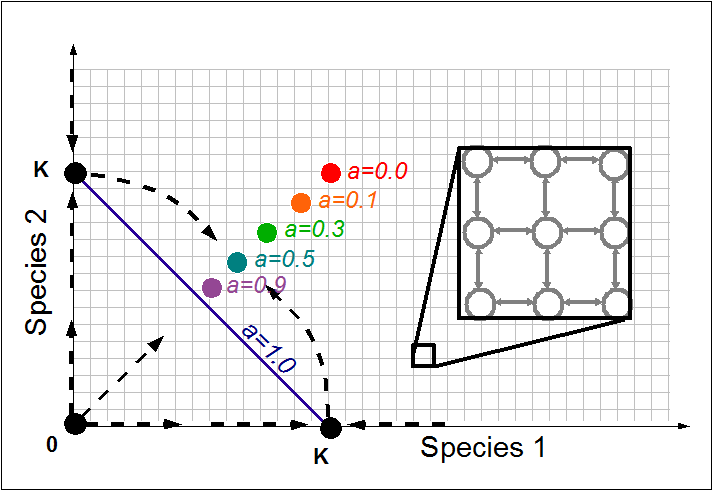
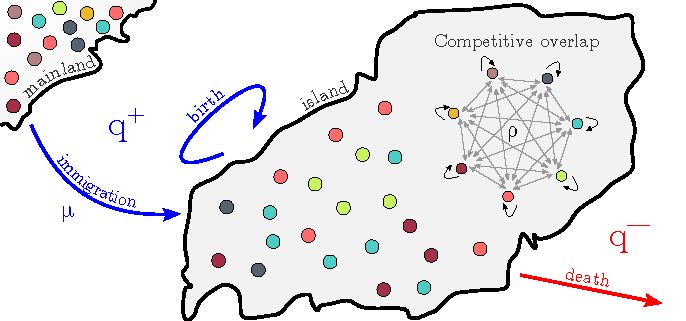
It has been progressively realized in the past decade that variability and stochasticity in biological systems in many instances play a fundamental role in shaping the system behavior, rather than being just “noise”. These issues feature prominently in our work on molecular transport and signaling, and arise not only on the molecular level but also in cell collectives. Inspired by a number of problems in immunology, ecology and health – such as in-host pathogen co-evolution, diversity of microbiomes in health and disease, and cancer progression, we have been working to elucidate the basic principles of coexistence and extinction in small populations of interacting cells and microorganisms. Applying the methods of modern stochastic processes and first passage theory allowed us to overcome several technical difficulties to advance the existing models into more realistic regimes. Together with the lab of Josh Milstein, we are currently building microfludic devices to test some of the theoretical models.

Biological Physics Group
Research