Fluorescent proteins are a powerful experimental tool for tracking the locations of biomolecules in living cells. Prof. Sarah Rauscher and PhD Student Liam Haas-Neill at the University of Toronto collaborated with Prof. Eitan Lerner at the Hebrew University of Jerusalem to extend the use of these proteins to report on local density in crowded environments (Joron et al., Nature Communications, 2023).
The Lerner lab discovered that the fluorescence lifetime of monomeric fluorescent proteins, including green fluorescent protein and mCherry, differ from their standard values in crowded environments, and are sensitive to the local density of their immediate surroundings. What this means is that these proteins can be used for more than just tracking — they can also report on heterogeneities in density. This was put to the test by Eran Meshorer’s lab, who used this approach to measure local densities within biomolecular condensates in embryonic stem cells.
The experiments in the Lerner and Meshorer labs established the utility of fluorescence lifetime imaging of fluorescent proteins for detecting heterogeneities in local density. However, it was not possible to infer the molecular mechanism for the observed changes in fluorescence lifetime using experimental data alone.
To address this question, Haas-Neill and Rauscher carried out molecular dynamic simulations of mCherry in solution and in the presence of a crowder (polyethylene glycol, PEG). Through a careful analysis of the resulting conformational ensembles, they found that PEG induces a subtle conformational change in the chromophore pocket of mCherry (see figure below).
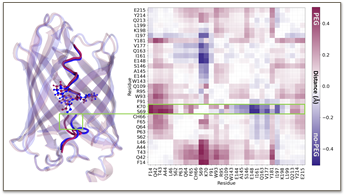
Conformational change in the chromophore pocket of fluorescent protein mCherry due to crowding. (a) Representative structures from the simulations showing helix conformations in the presence of PEG (plum) and without PEG (navy). (b) This map was obtained by subtracting the average C-alpha distance map of the simulations with and without PEG, revealing the subtle conformational change in the chromophore’s environment due to PEG crowding. (Joron et al., Nature Communications, 2023)
Increased crowding levels increase the degrees of freedom around the chromophore inside its β-barrel, which is sufficient to enhance the non-radiative de-excitation pathway and reduce the fluorescence lifetime. This collaborative study uncovered the molecular basis for the effect of molecular crowding on the fluorescence lifetime of monomeric fluorescent proteins, leading to a novel probe of local density in liquid-liquid phase-separated disordered states of proteins in vitro and in vivo.
Read the full publication here: https://www.nature.com/articles/s41467-023-40647-6
More information here: https://www.jpost.com/science/article-759824

