"Diffusion and Oligomerization States of the Muscarinic M1 Receptor in Live Cells: The Impact of Ligands and Membrane Disruptors" published in May 2024 in The Journal of Physical Chemistry

image: Prof. Claudiu Gardinaru, Xiaohan Zhou
Authors: Xiaohan Zhou, Horacio Septien-Gonzalez, Sami Husaini, Richard J. Ward, Graeme Milligan, Claudiu C. Gradinaru
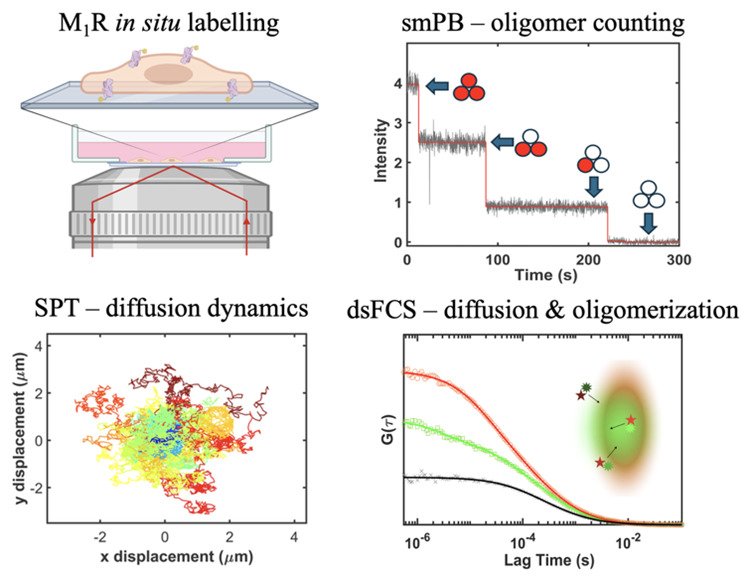
G protein-coupled receptors (GPCRs) are a major gateway to cellular signalling, which respond to ligands binding at extracellular sites through allosteric conformational changes that modulate their interactions with G proteins and arrestins at intracellular sites. High-resolution structures in different ligand states, together with spectroscopic studies and molecular dynamics simulations, have revealed a rich conformational landscape of GPCRs. However, their supramolecular structure and spatiotemporal distribution is also thought to play a significant role in receptor activation and signalling bias within the native cell membrane environment. Here, we applied single-molecule fluorescence (SMF) techniques, including single-particle tracking, single-molecule photobleaching, and fluorescence correlation spectroscopy, to characterize the diffusion and oligomerization behavior of the muscarinic M1 receptor (M1R) in live cells, with experiments performed in the presence of different orthosteric M1R ligands and of several compounds known to change the fluidity and organization of the lipid bilayer. M1 receptors exhibit diffusion characterized by three diffusion constants: immobile, confined, and Brownian, whose populations were found to be modulated by both orthosteric ligands and membrane disruptors. The lipid raft disruptor C6 ceramide led to significant changes for CD86, while the diffusion of M1R remained unchanged, indicating that M1 receptors do not partition in lipid rafts. The extent of receptor oligomerization was found to be promoted by increasing the level of expression and the binding of orthosteric ligands; in particular, the agonist carbachol elicited a large increase in the fraction of M1R oligomers. This study provides new insights into the balance between conformational and environmental factors that define the movement and oligomerization states of GPCRs in live cells under close-to-native conditions.
Interview with Xiaohan Zhou, first author of this research
Could you describe the primary focus of your research project? What specific objectives or questions are you aiming to address through your research?
My main research focus is about the spatiotemporal regulation of the GPCRs signalling process in live cells, to delineate where, when and how these receptors couple with the intracellular cognate G proteins and pass on the extracellular stimuli upon activation. While significant evidence from recent structural and spectroscopic studies suggests GPCRs exhibit a dynamic equilibrium between multiple conformational and oligomeric states, their spatial organization and temporal heterogeneity in the plasma membrane are also crucial for downstream signalling response and functionality. Based on the live cell single-molecule fluorescence system established in this research article, I aim to address the following questions: Is the coupling between M1R and G11 protein purely collisional, or do pre-coupled complexes exist (basal coupling)? Does coupling occur primarily within subdomains (lipid rafts) of the plasma membrane that are defined by the cytoskeleton filament mesh (the “picket and fence model”)? How does muscarinic ligands affect the stability of these complexes or bias the signalling pathway?
What methodologies or approaches are you employing in your research? Are there any innovative or unique aspects to your research methods?
The main technique that I employ is single-particle tracking (SPT), in which I use a custom-built total internal reflection fluorescence (TIRF) microscope to capture the motion of individual fluorescent labelled receptor molecules at the bottom membrane of live cells. With significant resolution and signal-to-noise ratio to visualize point spread functions from single emitters, the obtained spatial and intensity information from SPT can be used to characterize the complex behaviour of individual proteins as they explore different cellular micro-environments, interact with other macromolecules and form complexes with other proteins. I am currently working on implementing dual-color single-particle tracking (dcSPT) where one may simultaneously track two different species of proteins labelled with spectrally separated probes. Eventually I will combine this technique with Förster resonance energy transfer (FRET) to investigate fine detailed dynamics in proximity (1-10 nm), which has rarely been explored in this field of research.

Have you collaborated with other researchers or departments on your projects? How does your research contribute to interdisciplinary efforts within the department or beyond?
I have collaborated with laboratories both within and outside the university. In collaboration with the Prosser Lab (Biochemistry, UofT), we have been investigating the conformational dynamics of A2A adenosine receptor (another type of class A GPCR) and its interactions with the cognate Gs protein by combining SMF and nuclear magnetic resonance (NMR) techniques. Both in vitro and in vivo experiments that I perform provide real-time dynamic information about the protein system with significant sensitivity, complementing the atomic-level structural insights obtained with NMR. I am also collaborating with the Milligan Lab (Biology, University of Glasgow) on the signalling process of M1R. Funded by a Chemistry & Physical Sciences (CPS, UTM) Research Visit Fellowship, I have visited Dr. Graeme Milligan’s lab to learn the molecular biology techniques and various in vivo assays that are crucial for my research plans. The activity assays and bulk fluorescence experiments from their lab will provide corroborate results for my research on the initial steps of GPCR coupling.
How do you envision your research impacting the broader field or society? Are there potential real-world applications for your findings?
GPCRs are highly dynamic membrane proteins that use conformational flexibility and oligomerization to drive signalling via promiscuous pathways. Providing a solid quantitative foundation for this emerging paradigm requires carefully designed samples and adequate biophysical methods that can measure the full conformational distribution of states of the receptor. More importantly, those observations need to include the context of binding of ligands of varying efficacy, heterogeneous membrane architecture, receptor oligomerization and coupling to the various G protein transducer. A rich and diverse conformational landscape offers multiple avenues for novel therapeutic intervention, but without a clear mechanistic understanding to guide the process, the design of new drugs targeting GPCRs will be difficult. The applied in vivo SMF system also provides a platform for high-throughput screening drugs that regulate benevolent and/or malevolent downstream signalling pathways.
What challenges have you encountered during your research, and how have you worked to overcome them? Are there specific strategies or solutions you’ve found particularly effective?
As a Physics student, I have always been daunted and/or astonished by the complexity of the biology systems that I have been using. For instance, in order to perform SPT in live cells, the surface density of the receptors need to be at single-molecule, which is always hard to achieve due to the relative stochasticity of traditional transfection methods. To this end, I have to take advantage of a specially modified cell line that allows stable and controlled expression of receptors (Flp-InTM T-RExTM 293 cells) and modify various plasmids with molecular biology techniques, both learning curves of which proved to be steep. Furthermore, I have been working on simultaneously controlling the expression level of two different proteins in these cells in order to perform dcSPT, by integrating an Internal Ribosome Entry Site (IRES) based gene into the plasmid constructs, which is even hard for many biology labs. The main strategy that I employed is to get advice from experts in these specific fields and be active to ask proper questions whenever possible; critical thinking and paying attention to details matters much more than blindly working hard.
What are your plans for the future of your research? Are there specific goals you hope to achieve in the next few years?
The signalling process of GPCRs can be, and often is, promiscuous: the same receptor may couple to different G proteins or arrestins to initiate distinct pathways after activation by different ligands, while there has not yet been a general mechanism for this kind of biased signalling. Using current the muscarinic receptor system, I plan to probe the role that intracellular loop 3 (ICL3) plays in the downstream G protein selectivity, given the long disordered ICL3 of M1R (128 amino acids) forms extensive contacts with the coupling partners. The results will also provide insights into pharmacologically high-throughput screening of drugs that initiate various pathways of the same GPCR.
What advice would you give to other researchers in the department, especially those in related fields? Are there lessons learned from your research journey that you’d like to share?
Always be ready to step out of comfort zones. Biophysics is an interdisciplinary area that requires insights from various aspects; while it’s difficult to be proficient in every field, one should always be eager to absorb as much knowledge as possible in unfamiliar areas. Besides, science is all about critical thinking, one may never make significant progress without constantly being confronted with challenging questions and novel insights.
How do you engage with the departmental community, such as through seminars, workshops, or collaborative initiatives? Are there specific ways you encourage student involvement or mentorship in your research?
I try to attend both the weekly colloquiums of CPS at UTM and the Physics department at UTSG, and occasionally workshops on various topics. Given the relatively small CPS community, I engage with the departmental community, especially with fellow graduate students, more closely and frequently, even on daily basis. As a graduate student, I had the opportunity to supervise almost ten talented undergraduate students within the past three years, with most projects being successful (e.g. this research article). One key aspect, from my perspective, is to promote their research motivation and time engagement into these projects. There is no better encouragement than the desire for students to fulfill their self-driven interests, and getting timely feedback and communications from the supervisor.
Read the full paper here: https://pubs.acs.org/doi/10.1021/acs.jpcb.4c01035
More on Professor Claudiu Gradinaru's Group here: The Gradinaru Lab

